To understand this intricate process and develop effective therapies, researchers rely heavily on animal tumor models. These models play a pivotal role in mimicking human cancer in a living system, offering insights into tumor biology and serving as a platform for testing novel treatments.
This article explores the types, applications, and challenges of animal tumor models and their indispensable role in advancing cancer research.
What Are Animal Tumor Models?
Animal tumor models involve the use of living organisms—most commonly mice, rats, or zebrafish—to study cancer. These models simulate the growth, progression, and spread of human tumors, allowing researchers to investigate cancer biology in a whole-organism context.
Animal models are indispensable in cancer research because they can replicate the tumor microenvironment, immune interactions, and therapeutic responses, providing critical data before transitioning to clinical trials.
Types of Animal Tumor Models
1. Spontaneous Tumor Models
In spontaneous tumor models, animals naturally develop tumors due to genetic predisposition or exposure to environmental carcinogens. These models closely mimic human cancer progression.
- Example: Certain dog breeds, such as Boxers or Golden Retrievers, have a natural predisposition to cancers like lymphoma or osteosarcoma.
- Use Case: Ideal for studying the natural history of tumors and testing therapies in a setting that resembles human disease.
2. Induced Tumor Models
Tumors are artificially induced in animals using chemical, physical, or biological methods.
- Chemical Carcinogens: Substances like N-nitrosourea (ENU) or dimethylbenzanthracene (DMBA) induce site-specific cancers.
- Radiation-Induced Models: Exposure to ionizing radiation creates tumors, such as leukemias or solid malignancies.
- Oncogenic Viruses: Certain viruses, like the polyoma virus or murine leukemia virus, can induce tumor formation.
These models are valuable for understanding how environmental factors contribute to cancer initiation and progression.
3. Transplantation Models
Tumor cells or tissues are transplanted into animals to study cancer growth and therapeutic responses.
- Syngeneic Models: Tumor cells from the same species as the host are implanted, maintaining a functional immune system. These models are widely used in immunotherapy research.
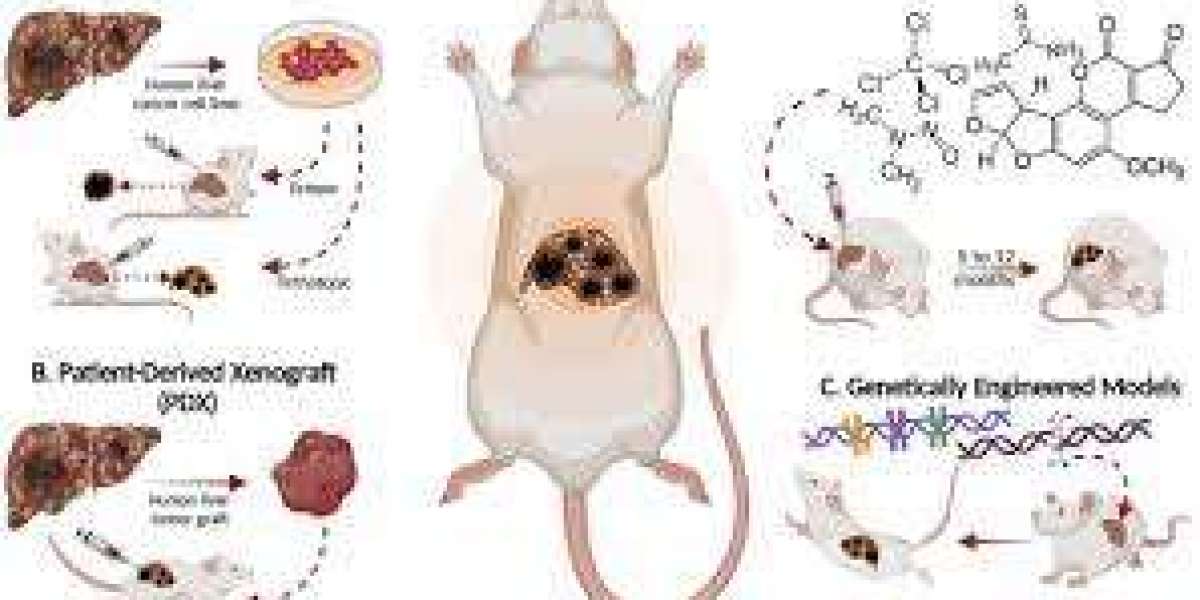
- Xenograft Models: Human tumor cells or tissues are implanted into immunodeficient animals (e.g., nude or SCID mice). Xenografts are critical for studying human-specific tumor biology and drug responses.
- Patient-Derived Xenografts (PDX): Tumors from cancer patients are directly implanted into animals. PDX models preserve the genetic and histological characteristics of the original tumor, making them a gold standard for personalized medicine research.
4. Genetically Engineered Models (GEMs)
In GEMs, animals are genetically modified to develop tumors due to specific oncogenic mutations or tumor suppressor gene deletions.
- Knock-In Models: Introducing mutations in oncogenes (e.g., KRAS, BRAF) to study their role in cancer.
- Knock-Out Models: Deleting tumor suppressor genes like TP53 or RB1 to investigate how their absence drives tumor formation.
- Conditional Models: Using systems like Cre-loxP to activate mutations in a specific tissue or at a specific time.
These models offer precise insights into the genetic and molecular mechanisms underlying cancer.
5. Humanized Models
Humanized models involve introducing human cells, such as immune cells or tumor tissues, into animals.
- Example: Humanized mice are used to study the interaction between human tumors and the immune system, especially in the context of immunotherapies like checkpoint inhibitors or CAR-T cells.
These models are vital for evaluating therapies targeting human-specific tumor or immune interactions.
Applications of Animal Tumor Models
1. Studying Tumor Biology
Animal models enable researchers to explore tumor initiation, progression, angiogenesis, and metastasis in a dynamic, systemic environment.
2. Preclinical Drug Testing
Before new drugs are tested in humans, they are evaluated in animal models to assess their safety, efficacy, and pharmacokinetics. Xenografts and GEMs are particularly valuable for these studies.
3. Immunotherapy Research
Animal models, such as syngeneic or humanized models, allow researchers to study immune system interactions with tumors and test therapies like immune checkpoint inhibitors, cancer vaccines, and CAR-T cells.
4. Personalized Oncology
PDX models provide a platform for testing therapies tailored to a patient’s specific tumor, advancing precision medicine.
5. Investigating Metastasis
Metastasis is a leading cause of cancer-related deaths, and animal models are essential for studying how tumors spread and identifying strategies to inhibit this process.
Advantages of Animal Tumor Models
Whole-Organism Context:
They replicate the interaction between tumors and other systems, such as the immune and vascular systems.Versatility:
Animal models are used for a wide range of studies, from understanding basic tumor biology to testing cutting-edge therapies.Translational Relevance:
While not perfect, these models often predict clinical outcomes better than in vitro systems alone.
Challenges and Limitations
Species Differences:
The biology of animals differs from humans, and findings in animal models don’t always translate to clinical success.Tumor Heterogeneity:
Human tumors are highly heterogeneous, making it difficult to replicate their complexity in animal models.Ethical Concerns:
The use of animals in research raises ethical issues, leading to strict regulations and a push for alternative methods.Cost and Complexity:
Developing advanced models, such as GEMs or PDXs, is resource-intensive.
Future Directions in Animal Tumor Models
Advanced Genetic Engineering:
CRISPR technology is enabling the creation of more precise and customizable tumor models.Integration with Non-Animal Models:
Combining animal models with 3D organoids, tumor-on-a-chip systems, and computational models will provide a more comprehensive understanding of cancer.Improved Humanization:
Enhancing humanized models to better mimic the human immune system and tumor microenvironment will increase their translational relevance.Ethical Alternatives:
Continued efforts are being made to refine, reduce, and replace animal use with alternative methods.
Conclusion
Animal tumor models remain a cornerstone of cancer research, providing invaluable insights into tumor biology and serving as critical platforms for therapeutic development. While no model is perfect, the ongoing refinement of these systems, coupled with emerging technologies, promises to enhance their relevance and accuracy in mimicking human cancer.
As we continue to advance our understanding of cancer, animal tumor models will remain integral to the development of innovative and effective treatments, bridging the gap between laboratory discoveries and clinical applications.